Do you know what is polymer resin used for? Do you know about polymer resin vs epoxy resin, acrylic polymer resin, photopolymer resin, or thermoplastic polymer resin?
Read this write-up to know more.
Polymer Resin Definition
The two components of a polymer resin are a liquid plastic resin and a liquid catalyst. The catalyst activates the liquid resin, starting the hardening process. The two components are blended completely after being mixed for a predetermined amount of time, typically in equal amounts.
Due to the chemical reaction between the resin and catalyst during mixing, it is common for the resin to heat up. The liquid can be poured over the surface that needs coating after being blended. The resin is self-leveling when it is liquid, which means that when it solidifies, it flows and creates a level surface.
Polymer Resin History
The resin family of plastics has been around for a very long time. Resins have historically been connected to manufactured synthetic materials or to natural products. Polymers, which are plastics, are synthetic resins.
A natural resin is sap. frequently observed as a thick, amber, gooey ooze seeping out of pine trees. Amber is created when pine sap fossilizes. Since ancient Egypt, natural resins have been used by humans. Around 1200 AD, the Mongols used resin to create a composite bow. They used pine resin to bind the components together.
The first synthetic resins were developed by scientists between the 1870s and the 1930s. Some of the most significant synthetic polymers became resins.
Basic Polymer Resin Types
Three main categories can be used to categorize polymer materials, some of which may overlap. By using a curing (or cross-linking) process, two of the groups—thermoplastic and thermosetting polymers—can be differentiated.

Above a particular temperature, thermoplastic polymers become more malleable and moldable and harden or re-harden upon cooling. When these polymers cool, they produce reversible bonds that enable cross-linked molecules to be broken and then reformed by additional heating. Because of this, completed goods made of thermoplastic materials are readily recyclable and reusable.
Heat or a chemical catalyst can irrevocably cure thermosetting polymers. Although they cannot be reused, they often offer more mechanical strength than thermoplastics. High-strength adhesives and parts utilized in high-temperature environments, such as automotive components, frequently use thermosetting compounds.
While elastomers are technically polymers with strong viscoelasticity, the name “rubber” is really used to refer to a specific subset of elastomeric materials. Usually made of carbon, hydrogen, oxygen, and/or silicon monomers, they are thermosets. Elastomers are amorphous, which means they can move widely and bend easily without breaking.
Polymer Resin Functions and Advantages Based on Various Factors
Here is the detail description of various types of polymer resin:
Category Based on Source
[1] Natural Polymers: Both plants and animals contain these polymers. Proteins, cellulose, carbohydrates, resins, and rubber are examples.
[2] Semi-synthetic Polymers: The most common examples of this subgroup are cellulose derivatives such as cellulose acetate and cellulose nitrate.
[3] Synthetic Polymers: Examples of man-made polymers include plastic (polythene), synthetic fibers (nylon 6), and synthetic rubbers (Buna-S).
Classification Based on Polymer Chain
The majority of synthetic polymers are organic; on the other hand, inorganic polymers typically have chains with no carbon atoms in them and are referred to as glass and silicone. Organic and Inorganic A polymer whose backbone chain is primarily made of carbon atoms is referred to as an organic polymer; the atoms attached to the side valencies of the backbone carbon atoms are, however, typically usually those of hydrogen, hydrogen, oxygen, oxygen, nitrogen, etc
Polymer Classification Based on Structure
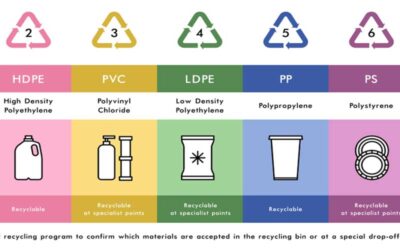
[1] Linear polymers are made up of lengthy, straight chains. Examples include PVC and high-density polyethylene. The majority of the time, linear polymers are generally soft, frequently rubbery materials that are prone to soften (or melt) when heated and dissolve in specific solvents.
[2] Branched Polymers: These polymers, like low-density polythene, comprise linear chains with some branches.
[3] Cross-linked polymers, such as vulcanized rubber, urea-formaldehyde resins, etc., are typically created from bi- and tri-functional monomers and have strong covalent connections between different linear polymer chains. In most circumstances, cross-linked polymers are solid and do not melt, soften, or dissolve.
Polymer Classification Based on Composition
[1] Homopolymer: A polymer formed when a single monomer is polymerized; a polymer that primarily consists of one kind of repeating unit.
[2] Copolymer: A copolymer is a polymer made up of two different kinds of monomers linked together in a single polymer chain.
Classification by Polymerization Mode
Polymers made by addition polymerization from a single monomeric species are known as homopolymers, such as polyethylene. Polymers made by addition polymerization from two different monomers are referred to as copolymers, such as Buna-S, Buna-N, etc. Addition Polymers: The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds, such as the formation of polythene from ethene and polypropene.
Condensation Polymers: These polymers are created by repeatedly combining two different bi- or tri-functional monomeric units. During these polymerization reactions, small molecules like water, alcohol, hydrogen chloride, etc. are eliminated. Examples of condensation polymers include terylene, terylene (dacron), nylon 6, 6, nylon 6, and others. For instance, nylon 6, 6 is created by combining hexamethylene diamine and adipic ac.
Classification Based on Molecular Forces
Intermolecular forces, such as van der Waals forces and hydrogen bonds, which are present in polymers and which also bind the polymer chains, control their mechanical properties. The polymers are categorized into the following groups according to the strength of the intermolecular forces that they contain:
Elastomers, fibers, liquid resins, thermosetting plastic, and liquid resins are only a few of the materials mentioned.
Polymerization of Composite Resin
By combining two or more organic monomer molecules, synthetic polymer chains are created. Polymers can be divided into two categories at the most fundamental level: copolymers, which combine more than one type of monomer, and homopolymers, which contain long chains of the same monomer. Either chain growth or step growth might result in polymerization.
In step-growth polymerization, two distinct monomer species react with one another. Molecular linking takes place all over the molecular matrix without the need for a separate initiator. Step-growth reactions also have a modest increase in molecular weight, the loss of specific monomers early in the reaction, and the continued activity of the chain ends after the process.
With chain-growth polymerization, growth normally takes place at one end of the chain and requires an initiator to create compound carbon bonds. In contrast to step-growth reactions, chain-growth reactions have a number of characteristics, such as an early, rapid increase in molecular weight (illustrated in the graph to the right), the permanent termination of the chain end following the reaction, and the presence of monomeric materials toward the end of the reaction period.
Where to Buy Polymer Resin?
Check out the best polymer resin reviews to find the perfect one for yourself. And Visit the resin store to buy resins online at a cheap price.
FAQ
1. Where can I use polymer resin?
Answer: Polymer resin can be used to create durable bottles and containers for items that need to be stored in sealed containers, as well as solid and elegant cases for pricey polymer resin powders, liquid polymer resin, and cosmetics.
2. What are the components of polymer resin?
Answer: The two components of a polymer resin are a liquid plastic resin and a liquid catalyst. The catalyst activates the liquid resin, starting the hardening process. The two components are blended completely after being mixed for a predetermined amount of time, typically in equal amounts.
3. What is the best between resin and polymer resin?
Answer: The resin family of plastics has been around for a very long time. Resins have historically been connected to manufactured synthetic materials or to natural products. Polymers, which are plastics, are synthetic resins.
Final Words
There are many polymer resins uses. Polymer resin can be used to create durable bottles and containers for items that need to be stored in sealed containers, as well as solid and elegant cases for pricey polymer resin powders, liquid polymer resin, and cosmetics (polymer resin examples: polymer resin for 3d printing, nail polish, etc).
Read the pillar article to learn about all types of Resin Definitions, History, Types, Functions, Advantages, Disadvantages, and FAQ.